The timber rattlesnake (Crotalus horridus) is regularly a species of interest among amateurs and experts. This species has the largest range of any rattlesnake and can be found from New England south to Florida, on the eastern coast of the United States, and west from Minnesota to Texas. This species is facing severe declines across its range due to habitat loss, overharvesting, and general persecution. While this species is protected across much of its range, these protections often vary by situational and temporal scales. For instance, in Pennsylvania there is an open season, with permits, during the summer to take one adult male timber rattlesnake. In other states, the take of timber rattlesnakes is permitted only if they present an immediate threat to life, though this is vague and often is not enforced.
This species was previously divided into two separate subspecies. The first being the timber rattlesnake, Crotalus horridus horridus, and the second being the canebrake rattlesnake, C. h. atricaudatus. The canebrake subspecies was recognized based on morphology differences such as scale row counts, an orange dorsal stripe that bisects the chevron pattern, a stripe along the face that extends from the eye to rear of the mouth, and a larger overall size. However, closer looks at these features found that they were variable across the range of C. horridus, with scale count differences more closely tied to sexual dimorphism. More recently, genetic works have shown that C. horridus is represented by distinct east-west populations but not along a north-south gradient, further supporting the idea that no subspecies should be recognized.
Across much of their range C. horridus is one of the largest species, commonly reaching up to four feet in length. However, records of specimens up to six feet have rarely been recorded. There exists a degree of sexual dimorphism in this species with males reaching greater lengths than females (Typically greater than 42 inches). This difference in size can be attributed to the combative nature of males, whom will engage one another for breeding rights to nearby females. Likewise, males can typically be identified by having 21 or greater subcaudal scales while females will have 20 or less.

There are typically three morphs commonly found in the wild: yellow phase, black phase, and canebrake phase. The canebrake coloring is indicative of low elevation populations, typically associated with the southern range of the species. The name comes from one of the former subspecies of C. horridus, the canebrake rattlesnake, C. h. atricaudatus, and presents with a cream background color with dark chevrons, typically with an orange stripe. The yellow and black color phases, based on color of the head, were previously thought to be tied to sex with males being black and females being yellow. Studies in Pennsylvania have since shown this to be a myth.
Likewise, it was thought that color morph was based on ontogeny, due to the black phase members of this species commonly being found at high elevations, though this was also later disproved. The works by Schaefer, 1969 also showed that color morph could be determined at birth and would only be reinforced with age. A color trait shared by all members of this species is the cream colored ventrum that is marked by dark speckling. Even in the darkest of individuals this species can be readily recognized from other species based on its morphology. C. horridus presents with large, triangular scales with a thick medial keel that stands out from other species.
As with nearly all rattlesnakes, this species has a rattle at the end of the tail that is formed from interlocking keratinous beads. A common myth is that beads can be counted for an idea of age, though beads are added after each shed. A snake may shed several times a year with neonates adding 2 to 5 beads in the first year. It has been suggested that a rough age can be determined by counting the number of rattle segments and dividing by the average number of sheds a snake may go through, however, this should only be utilized if the specimen has it’s button, the first segment, intact. Lastly, this species has a facial pit on either side of the head between the nostril and the eye. This organ allows the snake to sense heat emanating from the environment and potential prey. The posterior portion of the pit contains a membrane stretched across it. This membrane attaches to thermal receptors which are connected to the trigeminal nerve. This nerve feeds into the optic portion of the brain where it can be represented as visual stimuli.

This species is commonly found in forested habitat across its range. In northern portions of its range this species is strongly tied with rocky slopes within and along forested habitat. In the southeastern portions of the range, this species is commonly tied to forested flood plains, thickets, and swamps. To the west this species prefers dry, brushy flatlands and beech-maple-birch woodlands.
In the northern parts of the range this species hibernates in communal den sites to overwinter through harsh weather. A study of den sites in the northern ranges showed that 70% were on south facing slopes while the remaining 30% were southwest and southeast facing slopes. Likewise, members of the “canebrake” variety tend to overwinter in stumps and tree root systems for short periods of extreme cold.

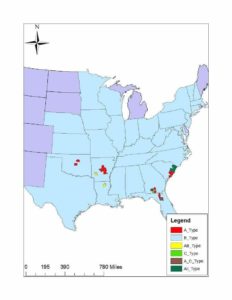
Like all vipers, the timber rattlesnake has a solenoglyphous dentition. That is, this species has large, retractable fangs that replace the maxillary teeth and fold against the maxillary bone. These fangs are attached to large venom glands by venom ducts. These fangs are often accompanied by a secondary set of fangs that will replace the primary set as wear and tear sets in. It has been shown that the venom composition of this species varies geographically with four variations: A, B, A+B, and C. Type A venom was found in the southern portion of the range, likely tied to the “canebrake” variant of C. horridus. This venom type is neurotoxic and contains canebrake toxin. Type B is found through the range of C. horridus, though primarily in the north. This venom type is primarily hemotoxic in nature. The third variant, type A+B, contained a mix of hemotoxins and neurotoxins and was found in areas when the two previously recognized subspecies intermixed. The last type, type C, was described by the author as being one of the weakest venoms the author has explored with a low LD50 in a lab setting, lacking both the canebrake toxin as well as the peptides that make type B potent. This last venom type was found in Georgia, Florida, and South Carolina and seems to be, at least partially, sympatric with type A (Glenn et al., 1994). It is unknown why this variation in venom exists in C. horridus, though similar studies in Crotalus scutulatus suggests variation in diet is the driving force.
This species is an ambush predator that often relies on fallen trees to locate prey. Fallen trees tend to act as travel corridors and basking platforms for many small fauna. In what has become known as the Reinert position, this species tends to curl up along fallen trees with a portion of the body and lower jaw meeting the log. This allows the snake to feel for vibrations of potential prey items, typically rodents. However, other small animals also make up at least some of the diet including members of the “Lacertilia”, Serpentes, Anura, Piciformes, Galliformes, Passeriformes, Chiroptera, and Eulipotyphla. The proportion of prey consumed is directly related to their relative abundance in the environment. It seems a majority of prey are captured at night.
While C. horridus is often thought of as being a top predator there are several species that will predate on them if the opportunity arises. In the northern part of the range, black racers, Coluber constrictor, are commonly found near den and gestation sites, and are known to take young C. horridus. Hawks may even prey on adult individuals. In the southern portion of their range both Drymarchon sp. and Lampropeltis sp. are known to prey on both large and small individuals of C. horridus, with Lampropeltis being immune to their venom and Drymarchon showing resistance.
Once the weather warms up and becomes more appropriate males, post-partum females, and non-breeding females will disperse from den sites into surrounding forest for feeding opportunities. In addition to foraging behavior, males will also seek out receptive females from surrounding subpopulations to breed with in late summer. Breeding will occur in late summer with females storing the sperm of males over winter. The following spring the eggs will become fertilized and females will give birth in late summer to early fall. There is at least some evidence that males will guard basking females or highly suitable basking habitat in hopes that receptive females will show up. Likewise, there is some evidence showing that males will protect gravid females and offspring.
Females of C. horridus seem to aggregate in family groups of related individuals. This lends several benefits including group defense against predators as well as increasing the ability to thermoregulate. It is theorized that not only does grouped basking increases the pressure against predators, but females may be more likely to defend a site if they know that related members will indirectly benefit. Likewise, if adults are grouped together it increases the likelihood that neonates may scent trail an adult to den sites, increasing survivability of offspring. C. horridus is strongly k-selected with females not maturing until roughly 4 to 10 years of age and only breeding once every 2-6 years, depending on abiotic conditions, while having relatively small litters of 3 to 16 young. This species is viviparous, giving birth to live young with at least some degree of nutrient transfer between more and offspring. Mothers will stay with their offspring for up to two weeks, roughly timing their parting with the first shed of the neonates. During this time, mothers will typically become bolder and actively defend the young against would-be predators. Surprisingly, neonate individuals tend to act in an opposite way, being incredibly curious to the happenings around them and not readily avoiding danger as they should (pers. obs.).

There are many biotic and abiotic factors that contribute to a population’s numbers and that may cause declines including habitat destruction, overharvesting, pollution, and disease (Wilcove et al., 1998). The factors described by Wilcove et al., 1998 all contribute to the population changes in C. horridus. Habitat destruction is an extensive problem that many species in the modern age are facing, imperiled or otherwise. Roads have become commonplace through many habitat types and have been shown to restrict gene flow and genetic diversity among population C. horridus, specifically, has been shown to be exceptionally susceptible to the impacts of roadways bisecting habitat because of their unwillingness to traverse open habitat. Studies have also shown that C. horridus is already experiencing a decrease in genetic diversity in the south due to population fragmentation by roadways.
Overharvesting has appeared, until recently, in the form of Rattlesnake Roundups. These events awarded prizes to participants in various categories such as largest snake and longest rattle. Of the snakes collected at hunts, a large number appeared to be gravid females, thought to have been collected in such numbers due to their preference for open areas with high amounts of sunlight. While hunters were supposed to return captured snakes to the same area they were captured, several hunters had explained they had no intention of doing so. It has been shown that C. horridus who have been relocated experience high mortality in the range of 50%. The relocation of snakes coupled with severe injury and handling of gravid individuals could potentially carry many unintended consequences. Fungal diseases have impacted many taxa such as: Batrachochytrium dendrobatidis in Anura, B. salamandrivorans in Caudata, Pseudogymnoascus destructans in Chiroptera, and Ophidiomyces ophiodiicola in Serpentes. There have been anecdotal accounts of O. ophiodiicola in Pennsylvania in Luzerne (LaDuke pers. comm., pers. obs.) and Lycoming (Dunning pers. comm.) counties. Additionally, there are anecdotal accounts that repeated handling or excess stress may cause infections of Snake Fungal Disease (SFD) to become more severe. However, with enough warmth and several molts it would seem that many individuals can overcome infections. The full impacts of Snake Fungal Disease are unknown but are one more reason that a species with a cryptic lifestyle and low fecundity should be monitored. In addition to these factors, hiking trails have become more abundant throughout the commonwealth. One study revealed a negative correlation between species abundance and trail area in wood turtles. The average person is largely biased against rattlesnakes and hiking trails through habitat increase the likelihood of human interaction with the species, leading to eventual mortality. Lastly, it seems that areas with high development in the form of buildings and roadways tend to have lower population numbers of C. horridus.

That being said, there is some positive news concerning this species. For instance, the Pennsylvania Fish and Boat Commission has studied timber rattlesnakes in Pennsylvania for over a decade and they strongly believe that Pennsylvania represents the strongest population of C. horridus in the northeast, with over 2000 sites recorded in the state. Other states seem to be enacting and increasing protections around this species. Thanks to social media with FaceBook groups, like Wild Snakes: Education and Discussion, and outreach programs, like the Pennsylvania State Atlas Project (www.paherpsurvey.org), it seems public perception has increased about wildlife in recent years. Hopefully we can keep up this momentum and continue to educate the general public and reduce unnecessary fear.
Works Cited
05.10 — Depredation Permit Requirement, apps.agfc.com/regulations/detail/0231d521-5e9d-4390-ab47-b04888ac6412/.
Adamski J. 2019. Quantifying the Effects of Habitat Disturbance on the Timber Rattlesnake, Crotalus horridus , in Pennsylvania. East Stroudsburg University, East Stroudsburg, PA.
Allender M.C., Raudabaugh D.B., Gleason F.H. & Miller A.N. 2015. The natural history, ecology, and epidemiology of Ophidiomyces ophiodiicola and its potential impact on free-ranging snake populations. Fungal Ecology. 17: 187–196.
Andrews K.M. & Gibbons J.W. 2005. How do Highways Influence Snake Movement? Behavioral Responses to Roads and Vehicles. Copeia. 2005: 772–782.
Blackburn D.G. 2000. Classification of the Reproductive Patterns of Amniotes. Herpetological Monographs. 14: 371–377.
Blehert D.S., Hicks A.C., Behr M., Meteyer C.U., Berlowski-Zier B.M., Buckles E.L., Coleman J.T.H., Darling S.R., Gargas A., Niver R., Okoniewski J.C., Rudd R.J. & Stone W.B. 2009. Bat White-Nose Syndrome: An Emerging Fungal Pathogen?. Science. 323: 227–227.
Campbell J.A. & Lamar W.W. 2004. The venomous reptiles of the Western Hemisphere. Comstock Pub. Associates, Ithaca. 2 pp.
Clark A.M., Moler P.E., Possardt E.E., Savitzky A.H., Brown W.S. & Bowen B.W. 2003. Phylogeography of the Timber Rattlesnake (Crotalus horridus) Based on mtDNA Sequences. Journal of Herpetology. 37: 145–154.
Clark R.W. 2002. Diet of the Timber Rattlesnake, Crotalus horridus. Journal of Herpetology. 36: 494–499.
Clark R.W., Brown W.S., Stechert R. & Greene H.W. 2012. Cryptic sociality in rattlesnakes (Crotalus horridus) detected by kinship analysis. Biology Letters. 8: 523–525.
Clark R.W., Brown W.S., Stechert R. & Zamudio K.R. 2010. Roads, Interrupted Dispersal, and Genetic Diversity in Timber Rattlesnakes: Roads and Population Genetics. Conservation Biology. 24: 1059–1069.
Clark R.W., Marchand M.N., Clifford B.J., Stechert R. & Stephens S. 2011. Decline of an isolated timber rattlesnake (Crotalus horridus) population: Interactions between climate change, disease, and loss of genetic diversity. Biological Conservation. 144: 886–891.
Ernst C.H. 1992. Venomous reptiles of North America. Smithsonian Institution Press, Washington. 236 pp.
Ernst C.H. & Ernst E.M. 2003. Snakes of the United States and Canada. Smithsonian Institution Press, Washington, D.C. 668 pp.
Furman J. 2007. Timber rattlesnakes in Vermont and New York: biology, history, and the fate of an endangered species. University Press of New England, Hanover. 207 pp.
Galligan J.H. & Dunson W.A. 1979. Biology and status of timber rattlesnake (Crotalus horridus) populations in Pennsylvania. Biological Conservation. 15: 13–59.
Garber S.D. & Burger J. 1995. A 20-Yr Study Documenting the Relationship Between Turtle Decline and Human Recreation. Ecological Applications. 5: 1151–1162.
Gibbons J.W. 1972. Reproduction, Growth, and Sexual Dimorphism in the Canebrake Rattlesnake (Crotalus horridus atricaudatus. Copeia. 1972: 222.
Gibbons W. 2017. Snakes of the Eastern United States. The University of Georgia Press, Athens. 416 pp.
Glenn J.L., Straight R.C. & Wolt T.B. 1994. Regional variation in the presence of canebrake toxin in Crotalus horridus venom. Comparative Biochemistry and Physiology Part C: Pharmacology, Toxicology and Endocrinology. 107: 337–346.
Gloyd H.K. 1935. The Cane-break Rattlesnake. Copeia. 1935: 175–178.
Gloyd H.K. 1940. The rattlesnakes, genera Sistrurus and Crotalus: a study in zoogeography and evolution. Society for the Study of Amphibians and Reptiles, Milwaukee. 266 pp.
Goris R.C. 2011. Infrared Organs of Snakes: An Integral Part of Vision. Journal of Herpetology. 45: 2–14.
Guthrie A.L., Knowles S., Ballmann A.E. & Lorch J.M. 2016. Detection of Snake Fungal Disease Due to Ophidiomyces ophiodiicola in Virginia, USA. Journal of Wildlife Diseases. 52: 143–149.
Hewlitt J.B. & Schuett G.W. 2019. Crotalus horridus (Timber Rattlesnake). Male Defense of Mother and Offspring. Herpetological Review. 50: 389–390.
Howey C. 2017. Defense of a Female Hotspot by a Male Timber Rattlesnake. Herpetological Review. 48: 16–19.
Hulse A.C., McCoy C.J. & Censky E.J. 2001. Amphibians and reptiles of Pennsylvania and the Northeast. Comstock Publishing Associates, Ithaca. 419 pp.
Klauber L.M. 1956. Rattlesnakes: Their Habits, Life Histories, and Influence on Mankind. University of California Press, Berkeley and Los Angeles. 1–708 pp.
Klemens M.W. 1993. Amphibians and reptiles of Connecticut and adjacent regions. State Geological and Natural History Survey of Connecticut, Hartford. 318 pp.
Lorch J.M., Knowles S., Lankton J.S., Michell K., Edwards J.L., Kapfer J.M., Staffen R.A., Wild E.R., Schmidt K.Z., Ballmann A.E., Blodgett D., Farrell T.M., Glorioso B.M., Last L.A., Price S.J., Schuler K.L., Smith C.E., Wellehan J.F.X. & Blehert D.S. 2016. Snake fungal disease: an emerging threat to wild snakes. Philosophical Transactions of the Royal Society B: Biological Sciences. 371: 20150457.
Martel A., Spitzen-van der Sluijs A., Blooi M., Bert W., Ducatelle R., Fisher M.C., Woeltjes A., Bosman W., Chiers K., Bossuyt F. & Pasmans F. 2013. Batrachochytrium salamandrivorans causes lethal chytridiomycosis in amphibians. Proceedings of the National Academy of Sciences. 110: 15325–15329.
Martin W.H. 1993. Reproduction of the Timber Rattlesnake (Crotalus horridus) in the Appalachian Mountains. Journal of Herpetology. 27: 133.
McBride M.P., Wojick K.B., Georoff T.A., Kimbro J., Garner M.M., Wang X., Childress A.L. & Wellehan J.F.X. 2015. Ophidiomyces ophiodiicola dermatitis in eight free-ranging timber rattlesnakes (Crotalus horridus) from Massachusetts. Journal of Zoo and Wildlife Medicine. 46: 86–94.
Pisani G.R., Collins J.T. & Edwards S.R. 1973. A Re-Evaluation of the Subspecies of Crotalus horridus. Transactions of the Kansas Academy of Science (1903-). 75: 255.
Reinert H.K. 1984a. Habitat Separation Between Sympatric Snake Populations. Ecology. 65: 478–486.
Reinert H.K. 1984b. Habitat Variation Within Sympatric Snake Populations. Ecology. 65: 1673–1682.
Reinert H.K. 1990. A profile and impact assessment of organized rattlesnake hunts in Pennsylvania. Journal of the Pennsylvania Academy of Science. 64: 136–144.
Reinert H.K., Cundall D. & Bushar L.M. 1984. Foraging Behavior of the Timber Rattlesnake, Crotalus horridus. Copeia. 1984: 976–981.
Reinert H.K. & Rupert R.R. 1999. Impacts of Translocation on Behavior and Survival of Timber Rattlesnakes, Crotalus horridus. Journal of Herpetology. 33: 45–61.
Retallick R.W.R., McCallum H. & Speare R. 2001. Endemic Infection of the Amphibian Chytrid Fungus in a Frog Community Post-Decline. Biological Conservation. 97: 331–337.
Rubio M. 2014. Rattlesnakes of the United States and Canada. BookBaby, Cork.
Schaefer G.C. 1969. Sex independent ground color in the timber rattlesnake, Crotalus horridus horridus. Herpetologica. 25: 65–66.
Stechert R. 1982. Historical distribution of timber rattlesnake colonies in New York State. HERP: Bulletin of the New York Herpetological society. 17: 23–24.
“Summary Book – Reptiles & Amphibians.” Pennsylvania Fish and Boat Commission, pfbc.pa.gov/fishpub/summaryad/repamp.html.
Sutherland I.D. 1958. The “combat dance” of the Timber Rattlesnake. Herpetologica. 14: 23–24.
Wilcove D.S., Rothstein D., Dubow J., Phillips A. & Losos E. 1998. Quantifying Threats to Imperiled Species in the United States. BioScience. 48: 607–615.
Zancolli G., Calvete J.J., Cardwell M.D., Greene H.W., Hayes W.K., Hegarty M.J., Herrmann H.-W., Holycross A.T., Lannutti D.I., Mulley J.F., Sanz L., Travis Z.D., Whorley J.R., Wüster C.E. & Wüster W. 2019. When one phenotype is not enough: divergent evolutionary trajectories govern venom variation in a widespread rattlesnake species. Proc. R. Soc. B. 286: 20182735.